DQ, IQ, OQ, PQ In FDA-Regulated Industries: The Complete FAQ Guide In 2025
DQ, IQ, OQ, PQ In FDA-Regulated Industries: The Complete FAQ Guide In 2025
Have you ever heard of DQ, IQ, OQ, or PQ? In FDA-Regulated industries, every piece of equipment and system is hooked to proper regulations. This means each step and protocol is jotted down in specified protocols. Why is it required to have verified documentation? What happens if your equipment is missing functional specifications?
In this article, we have talked about from basic to advanced literature about DQ, IQ, OQ, PQ in FDA-regulated industries. Stay on this page if you have less time and more questions in mind. We have explained the topic with interactive illustrations and examples to make you learn about these important certifications required in FDA regulated industries.
1.What are DQ, IQ, OQ, PQ In FDA-Regulated Industries?
DQ, IQ, OQ, PQ in FDA-Regulated Industries
To understand the answer, you should first grasp what are FDA regulated industries. Actually, FDA is mainly meant to ensure safety and protection. Especially when it comes to dealing with medicines, biologicals, and items associated with health and medicine. Therefore, it is particularly important when you’re producing goods for foods, humans, pets, etc.
So, the FDA or Food and Drug Administration USA has regulated some essential rules and regulations; and is responsible for maintaining them.
In the pharma industry, you cannot deny accuracy and safety. You should know that any slight mishandling or error can cause potential hazards to patients. Subsequently, the reliability of equipment is paramount. DQ, IQ, OQ, PQ in FDA regulated industries is providing a promising way that certifies the current equipment and procedure is compiled with reliable performance and results and ensuring client safety.
2.What is DQ?
DQ- Picture Courtesy: MGA Techonology
This is ‘design qualification’ which is utilized in several industries like pharmaceuticals. It ensures that the system design is exactly according to regulated specifications. The DQ is mainly carried out when you are at the beginning of any project development; that promises you the machine is up to the mark with high-quality standards.
The DQ is associated with deep evaluation of design documentation involved with specifications and structural framework of the equipment. Hence it certifies that the design is complete with all possible essential documentation.
The prime objective of DQ is to immediately identify the structural flaws of equipment. Side by side, it ensures that equipment is compliant with FDA regulations and the establishment of the records relevant to the unit.
It is involved with the needs of users as well as user specifications. That requires a detailed design format with review and approval of the machine by various personnel such as engineering teams, quality analysts, and stakeholders. Test and simulation and risk analysis (safety features), record keeping, and final approval.
3.What is IQ?
Installation qualification- Picture Courtesy: Access Industrial Solutions
So, once you confirm that the design qualification of equipment is up to the mark therefore the next task is the installation of the unit. Do you know how your system implements the experimentation? It is all based on the right installation.
FDA-regulated industries mainly focus on IQ whenever install a piece of new equipment and it is called an ‘Installation Qualification’ or IQ. It is actually a verification document that certifies that your system is qualified and configured as it is promised in its specification. For that, you can mostly refer to the installation checklist mentioned in the manual of the machine-generated by the manufacturer.
Moreover, the IQ that is involved with methods should be well-documented and included in the ‘Validation Master Plane’ or VMP.
The compulsory steps involved for IQ are mainly outlining the methods and conducting assessments. That is mainly involved with the verification of the machine and things associated with it. It is included with the specifications given by the manufacturer. Moreover, the installation conditions such as area and temperature around the equipment, electrical essentials, and software installation.
4.What is OQ?
Operational Qualification- Picture Courtesy: Tien Tuan Pharmaceutical
Whenever you check IQ, it is parallel to performing the OQ or operational qualification of the equipment. As its name shows, it is an assessment to analyse the operational performance of the equipment as per your needs. The purpose of this verification is to ensure that whatever your manufacturer claimed is really performing in your functional range. This means it is mainly performed to identify the properties of the unit and ensure its seamless operation.
To perform this test, the plan sheet provided by the manufacturer or regulated body is properly checked and accepted by the facility once the test is successfully conducted.
The essential steps for OQ involve a thorough inspection of the unit. It means, the inspection of software and hardware, how to start and run the test, and assessing the safety protocols. Moreover, the test run is conducted to ensure that the machine is providing you the fruitful results hence you can confidently run the machine hereafter.
5.What is PQ?
Performance qualification- Picture Courtesy: Contract Pharma
This is the final step which is assuring the absolute functional capability of the equipment. The performance qualification or PQ promises you that the unit will offer a consistent output for a long period of time.
The pharmaceutical industry considers PQ as a major asset. Consistency and high throughput are what every pharma looks for to generate high-production batches and meet healthcare needs. Therefore, the test ensures that the functionality, installation, and efficacy of the machine are up to regulatory standards.
6.Why DQ, IQ, OQ, PQ In FDA-Regulated Industries is essential?
Every FDA-regulated industry like medicine, pharmaceuticals, research, biologicals, and others cannot work without addressing specified compliance requirements. So, there are multiple reasons to implement DQ, IQ, OQ, PQ in FDA-regulated industries. What are those points? We have discussed them below so have a look at them.
You Can't Ignore FDA regulations
FDA regulated Protocols
As per FDA statement in 21 CFR Title 211.63, the equipment utilized for processing, packaging, and overall manufacturing of medicinal items must be in accordance with the correct in structure, and sizes, and up to suitability of the facility to bring operation and for cleaning as well as maintenance. So, it is the responsibility of the manufacturing company to bring you unit with up-to-the-mark features.
It should be provided with detailed instructions and usage properties. That includes how to operate, clean, and maintain the unit to give it an effective application.
Make Patients Safe & Ensure Quality Product
Safe and quality preparation- Picture Courtesy: Makinew
When you’re manufacturing any medicine or product in your plant; slight mishaps, mishandling, or problems in the unit can lead to severe consequences for you and the patient. This is commonly referred to that equipment which is new without any history. Therefore DQ, IQ, OQ, PQ in FDA regulated industries make you pretty safe from failure in performance or error in the making of medications.
It creates everything in well-documented pieces of evidence and verifies the hardware and software of the units. So, what’s next? It makes lesser chances of incorrectness and makes you ensure that equipment is passed with design till its final performance with reproducible output. Thus, you can ultimately get a good quality product.
Your Facility is Compliant
Quality standards- Picture Courtesy: Microsoft news
Every pharmaceutical industry must be compliant with FDA regulations. Following the documentation for equipment installation meets all needs that are set up by FDA. The verification for DQ, IQ, OQ, PQ in FDA regulated industries ultimately minimizes the chances of batch withdrawal or recalls and saves your budget.
No Failure and Early Management
Minimize interruption- Picture Courtesy: WIPO
Every manufacturing industry keeps processing its protocols 24/7. Hence, there are high chances of failure. The strict adherence of DQ, IQ, OQ, PQ in FDA regulated industries helps you in the identification of issues at the beginning.
When you consider DQ, it assures you that the purchase unit is strictly designed with quality standards. Similarly, IQ, OQ, and PQ assessments are thorough testing assuring everything about the processing and future protocols.
Therefore, once you’re well aware of every stage will have high confidence in operating, identification of problems, and early troubleshooting.
Record Keeping
Verified data- Picture Courtesy: Vaisala
Implementation of DQ, IQ, OQ, PQ in FDA regulated industries allows verified records related to equipment from its making to processing. You can easily trace the history required for various activities in pharmaceuticals and related companies. So, you can have a clear picture with easily identifiable issues about the unit.
Offering Good Training and Knowledge
Good knowledge- Picture Courtesy: Technopharm
Bringing a new setup into your facility will allow your employees to facilitate training and gain good knowledge during the DQ, IQ, OQ, PQ approach. By this, a group of workers understands the protocols, relevant troubleshooting, and extensive knowledge that ensures your team is well aware of the work process and handling.
Good relationships with Vendor
Good relationships with vendor- Picture Courtesy: Pharmaceutical Technology
During DQ, IQ, OQ, PQ, and verification, you may discuss general matters and discussion about equipment closely. It directly opens the gateway between you and the vendor and can carry it out for long-term professional relationships.
7.What type of sectors utilizes DQ, IQ, OQ, PQ In FDA-Regulated Industries?
You cannot ignore the implementation of DQ, IQ, OQ, and PQ in FDA-regulated industries. What are those industries? Let's discuss how each of these plays a significant role with examples accordingly.
Pharmaceutical Industry
Pharmaceutical equipment- Picture Courtesy: Korber Pharma
This is the major core to produce medicines. Whether oral, parenteral, topical, or powder medicines; a minor error can result in risky outcomes. It directly affects your expenditure and delay in processing. Therefore, whenever a new set of equipment is installed in a pharma facility.
The series of tests DQ, IQ, OQ, and PQ are followed to ensure conformity with regulatory standards. This general qualification activity guarantees that the manufacturing process satisfies all requirements and provides you with quality products.
Biotechnology Industry
Biotechnology assays- Picture Courtesy: faCellitate
In the biotechnology industry, absolute working is needed when dealing with delicate assays, sampling, and cell culture procedures. The necessities for DQ, IQ, OQ, PQ are paramount as the functioning equipment for biotechnology tasks ensures reliable and efficient performance.
Due to conduction with sensitive testing related to genomic alteration, molecular level, cellular level, and blood sampling in biotechnology; the safety, effectiveness, and purity must be maintained by strict following up of FDA regulations.
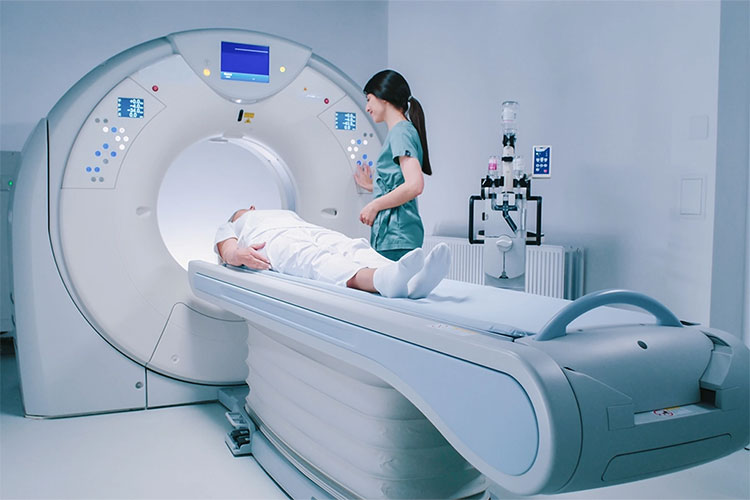
Medical Devices Industry
Medical Devices Industry- Picture Courtesy: SJRA
It is essential to qualify the equipment used for manufacturing and packaging procedures for the medical devices industry. However, under real conditions of production, it will deliver medical devices and parts with high quality, thereby, the products manufactured will be totally safe for patients and end-users. For example, X-ray machines, CT- Scanner, implantation devices, or radiology medical equipment.
Food Industry
Food industry- Picture Courtesy: Redline
In the food sector, safety related to raw ingredients, manufacturing, filling, and packaging plays a significant role. For this, your equipment must have adhered to DQ, IQ, OQ, and PQ documentation to ensure the system is well-functioning and compliant with regulatory requirements.
Nutraceutical Industry
Nutraceutical Products- Picture Courtesy: Market Research Intellect
Just like pharmaceuticals and food, the nutraceutical industry also undergoes strict testing and production of goods related to human consumption. Most of us the equipment used in nutraceuticals is derived from natural sources that offer wellness to you.
Therefore, DQ, IQ, OQ, and PQ confirm that the design of the machine conformed with all the requirements and conditions needed for manufacturing products under the required conditions.
Veterinary Industry
Veterinary Industry
There is a surging need for good quality veterinary products nowadays. This field of production is equally important as the above-mentioned FDA-regulated industries. The life and health of animals must be dealt with by medicines or energy-boosting products that are manufactured with strict conformities by regulatory bodies.
The DQ, IQ, OQ, and PQ documentation significantly emphasized that the current units used for the preparation of products, such as solid dosage, parenteral, or liquid formulation for your animals are safe and prepared by FDA-approved equipment.
8.How to execute DQ, IQ, OQ, PQ In FDA-Regulated Industries?
Typically, the pharma and related FDA-regulated industries focus on the following steps for the execution of DQ, IQ, OQ, PQ. For instance:
DQ
Design qualification- Picture Courtesy: GEA
DQ is mainly verified by the following three steps.
| Step 1 | Here, it involved double checking of ‘purchase order’ as well as the ‘user requirement specification’. In many cases, the purchase order is also termed as a ‘request for purchase’. The purpose of this step is to ensure that manufacturers agree to offer the following properties, specifications, and materials are mentioned in the document and well-engineered. |
| Step 2 | This section is hooked to detailed design specifications of the equipment that must be compliant with the drawing as presented to their customers and provided with updated features. |
| Step 3 | You can term it as the final step where you can have a deep inspection of the overall presentation of the product and prepare a summary related to the equipment. |
IQ
Installation Qualification
The IQ process is verified by the following steps:
| Step 1 | In this step, you will need to check the identification of the new equipment that is involved with the series, model number, type, automation, brand, etc. |
| Step 2
|
It is involved with the series of equipment and the qualification system as the installation requirement provided by the manufacturer's specification. |
| Step 3 | The entire favorable condition is verified by checking the temperature of the installation area, measuring the humidity level, monitoring the electrical connections as well as assessing the machine’s calibration. The final report is prepared once all evaluation is done. |
OQ
Operational Qualifications
OQ verification is performed to ensure that the equipment is operating properly as specified by the manufacturer. There are the following steps to make a successful OQ processing.
| Step 1 | The detail of the system, its software and hardware, and its operation is evaluated to check if the operational specifications is compliant with FDA regulations. |
| Step 2 | The step-by-step running of the machine is conducted out and various operating tests are carried that are highlight operation with surrounding conditions. Here, you can also create intentional failure situations and simulate the error modes to learn teamwork with troubleshooting for unexpected circumstances. |
| Step 3 | The result is summarized by observing all major details and possible recorded deviations and the corrective actions. The elaboration of each step in OQ validates that the unit is reliable and highlights the various risks and handling to enhance operational comprehensiveness. |
PQ
Performance qualification- Picture Courtesy: Chemtech
The performance qualification steps are:
| Step 1 | The real-time assessing tools are techniques are implemented to monitor the data by PQ execution, here you can integrate various sensors as well as utilize software to analyze critical parameters. |
| Step 2 | Furthermore, process analytical technology or PAT is offered to assess the quality and performance of the system. |
| Step 3
|
Also, the process capacity and design protocols are considered to evaluate the final limit of the production requirements. For example, if your recent production is 50, so in the future if it reaches above 100 then the PQ conduction test would verify that the process would be successful without affecting the quality of products. |
9.What are the challenges of maintaining and designing DQ, IQ, OQ, PQ In FDA-Regulated Industries?
With DQ, IQ, OQ, PQ In FDA-regulated Industries' usual strategies, you also encounter some challenges. We have discussed some major issues and their solutions.
Inadequate Planning and Scope Definition
Complex planning and scope
| Problem | Solution |
| One of the major issues in initial planning is vague or ambiguous requirements to be addressed by qualification, which most often brings gaps in documentation and compliance.
Now, a very vague scope can introduce uncertainty in particular validation needs, and such things may eventually be left out of the qualification process. therefore, stress over scope definition and clarity of requirements. Teams often compromise full documentation in order to meet very tight deadlines and miss all the critical steps. |
Precise Requirements Definition Engage with stakeholders as early as possible—quality assurance, engineering, and operations teams—to make sure that all requirements are clear and complete from the beginning so that all aspects of the validation process are understood by everyone involved.Clean Scope Documentation Develop a Validation master plan (VMP) that defines the scope of qualification, objectives, timelines, and contact responsibilities for each phase of qualification. The plan is supposed to be a roadmap that will cover the topic clearly and result in full compliance with standards that have been laid down by regulatory bodies. Clearly defining the scope and involving stakeholders leads to reduced risks of compliance and documentation gaps for companies. |
Requirement and Fulfilling of each Phase
Utilization of regulation- Picture Courtesy Pharmaceutical Processing world
| Problem | Solution |
| Each qualification including DQ, IQ, OQ, and PQ has different requirements and expectations, thus making the general process somewhat complicated. Since regulatory bodies like the FDA have to issue constant upgrades, it can get complicated for organizations to keep up with new norms.
Standards and guidelines of regulatory agencies, which are very often updated, may leave some gaps in qualification as companies cannot update fast enough.
|
Risk-based approach DQ, IQ, OQ, and PQ efforts should highlight the critical parameters and processes. Therefore, most risks need importance in the consideration that it complies with and is safe for the product being produced.Continuing Education and Training Regular and continuing training programs should be implemented so that all personnel will be current on all regulatory requirements, best practices, and new or additional standards or guidelines issued by agencies such as the FDA. Regular regulatory review |
Data and Documentation Management Challenges
Data and Documentation Management
| Problem | Solution |
| The DQ, IQ, OQ, PQ may generate huge amounts of data, making it very hard to manage accurately and in a timely manner, hence increasing risk for errors and delay in compliance.
Heavily documented needs can sometimes be incomplete, inconsistent, or simply inaccurate records. The lack of information about suppliers and manufacturers, coupled with the validation process, has hollowed credibility as well as compliance.
|
Advanced-Data Management Tools
Think then about the modern data management solutions that would simplify collection, storage, and analysis. Even the usage of electronic systems must improve accuracy, tracking, and reporting for requirements. Document Management System Secondly, a comprehensive document management system should be adopted and standardize formats of documents automatically, whereby all documentation processes are automated. This minimizes the chance for error and increases consistency, further simplifying retrieval and review during audits or regulatory checks. |
Inadequate or Missing Reports
Report issues- Picture courtesy: Zamann pharma
| Problem | Solution |
| Reports may be incomplete or lack clarity, hindering effective decision-making and compliance verification.
|
Report preparation templates
Implement the use of templates whereby all reports have full details, easy to communicate, and are in similar formats for easier understanding. |
Inconsistent Systems and Protocols
Inconsistent Systems and Protocols- Picture Courtesy: EESS
| Problem | Solution |
| In the present system, different departments follow one single procedure for validation. Most equipment types lack standard operating procedures for qualification and their results vary. It means that either the protocols are vague, wrong, or incoherently defined. Thus, inaccuracies are introduced within the qualification process. | Standard operating procedures (SOPs) Standardize all the SOPs. This shall include qualification for a uniform process and documentation across this will certainly assure consistency and rule out errors from the fragmented processes. |
Continuous Monitoring and Review
Continuous Monitoring and Review
| Problem | Solution |
| Maintaining control and oversight overqualified systems can be challenging, leading to potential lapses in compliance.
|
Standard Review Procedures
Implement a process of continuous monitoring and auditing so that the qualified system continues to remain in compliance with regulatory standards and operational requirements. |
Conclusion
The path of adding new equipment in your medical facility, pharmaceutical, biotechnology, etc is absolutely striking with challenges. But if you follow the right strategy and ideal resources that are compliant with FDA regulation can limit the obstructions. We would suggest if you have any problem or need to seek some advice, then always approach the right solution and experts who can solve the complexities during the regulatory issues. Why not contact us? We are a team of advisory professionals and manufacturers who strive for your support and guidance. For more information or FDA-regulated equipment for your production line, contact the Allpack team now.
Don't forget to share this post!
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 7101 8586
The Buyer's Guide

DQ, IQ, OQ, PQ In FDA-Regulated Industries: The Complete FAQ Guide In 2025 Read More »