Importance of Packaging Machine in the Pharmaceutical Industry
Importance of Packaging Machine in the Pharmaceutical Industry
Have you ever wondered the sanitary and efficiency of your medicines while you make the purchase in pharmacy? The various packaging machines in the pharmaceutical industry make the important work to convey the clean and efficient medical products to many patients.
Have you ever wondered the importance of packaging machine in pharmaceutical industry? Do you know the frequently used packaging machines in the pharmaceutical industry? How to find your suitable packaging machine in pharmaceutical industry? Here is the answers about all above questions.
1.What IsPackaging Machine In The Pharmaceutical Industry?
Pharmaceutical Packaging-Sourced:arka
For the pharmaceutical products, the unqualified and normal packaging may lead the damage of products in transporting and change effect for medical use. Pharmaceutical packaging is the special packaging for the reliable and sanitary protection of medical products and other products for pharmaceutical use.
The packaging machines in the pharmaceutical industry have various type and strict standard for the production of hygienic and protective pharmaceutical products. For different type and dosage, the packaging methods are different and you may see the various types of packaging machines in the pharmaceutical industry.
2.What Are The Common Packaging Material For Pharmaceutical Packaging Use?
For different packaging manner, there is different packaging material adopted to pharmaceutical packaging use.
Plastic
Plastic Material-Sourced:stringentdatalytics
Plastic material is one of the most common packaging material for pharmaceutical use. You may see the bottle, sachet, suppository and so many material for pharmaceutical use. Plastic material is cheap and can make the efficient and protective packaging for various types of material.
Glass
Glass Material-Sourced:glassproduct
Glass is the irreplaceable material for injecting medicine packaging. Its durability and great resisting to acid and alkalis make it also the important packaging material to some special medical products. Ampoule, vial and many medical packaging all need the application of glasses in it.
Aluminum
Aluminum Material-Sourced:aluminumfoil
Aluminum can be frequently found in blister packaging in pharmaceutical industry. The softness, flexibility and durability of aluminum make it the ideal packaging material for pack the capsule, soft gel and many other medical products. You may also see its use in sealing tube and so on.
Paper
Paper Material-Sourced:cdnbestsuppliers
Paper is usually used for sealing small sachet. The low price and anti light make it the great material for many medical products. And most importantly, it can make the sustainable and bio degradable work. For the final tertiary packaging, it can also makes the great carton for your medicine packaging.
3.What Are The Packaging TypesFor The Pharmaceutical Industry?
As there are various types of medicines in pharmaceutical industry, there are also various packaging types for you to choose. Here are the representative packaging types for pharmaceutical industry.
Blister packaging
Blister Packaging-Sourced:sedpharma
Blister packaging is one of the most common packaging type for pharmaceutical industry. For various tablet, capsule and soft gel, it can make the safe, air tight and protective packaging. The fragile products like medical devices or equipment can also be protected well from blister packaging.
Bottle packaging
Bottle Packaging-Sourced:dynamicmedia
Bottle packaging has a wide range. You may find the bottle packaging of various type from small bottle and big bottle. From the packaged material, it can also differed greatly. Which is your packaged material, syrup, air drop or other? The different packaged material should apply the different packaging machine type.
Sachet packaging
Sachet Packaging-Sourced:aranow
Sachet packaging is also a widely applied packaging type. The packaging of medical products into the sachet is cost effective and protective. The medical and herb powder is one type which adopts the sachet packaging most frequently. For granule, powder or some liquid sachet packaging is the recommended packaging way.
Tube packaging
Tube Packaging-Sourced:mplusa
Tube packaging is the packaging which suits the topical used medicines like cream, ointment, lotion and so on. The tube packaging can prove the efficiency and sanitary of packaged material greatly. And it can also make the more accurate and precise caring for some small zone for your skin or other places.
Suppository packaging
Suppository Packaging-Sourced:saintyco
Suppository packaging is the special packaging to make the suppository. Its packaging manner is different and makes the great benefits to some special medicine. Suppository gets into people’s body from rectum, vagina or urethra and melt when meeting your body temperature.
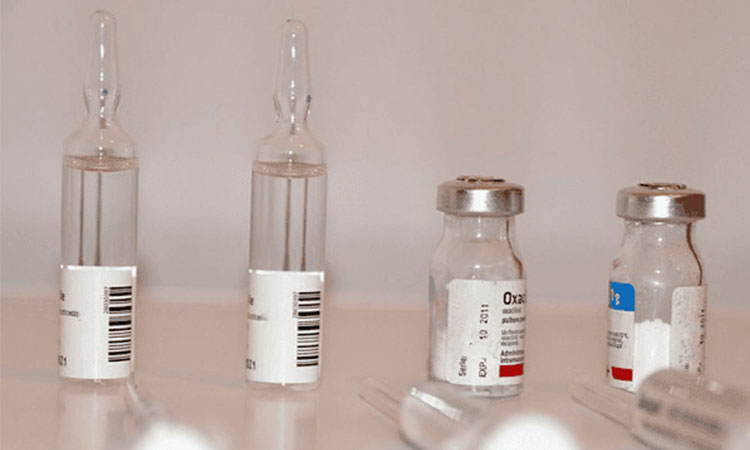
Vial and ampoule packaging
Vial And Ampoule Packaging-Sourced:uskbalaji
Vial and ampoule packaging is the packaging which is widely used in injection area. The medicine injection should be with the highest hygienic standard. And now the vial and ampoule packaging can also be used in wound caring, skin caring and so on for its great feature in sanitary and conserving.
4.What Are The Representative Packaging Machines In The Pharmaceutical Industry?
The pharmaceutical packaging machines are various. And here are some representative packaging machines which are used in pharmaceutical industry.
Blister packaging machine
ALLPAK Blister Packaging Machine
Blister packaging machine usually packs the capsules and tablets to make the airtight clean environment for the various medical products. Besides the common capsule and tablet, you may also see its use in medical equipment. It can make various form and size for the different need and requirement.
Strip packaging machine
ALLPAK Strip Packaging Machine
Strip packaging machine is used in pharmaceutical industry to pack the pill, tablet, capsule and so on. The preventing of pollution in the single strip intervals makes sure the great effect and sanitary of your medical products. The machines can pack the strip with high efficiency and great flexibility and you may also see its use in food, cosmetic and other industry.
Liquid filling machine
ALLPAK Liquid Filling Machine
Liquid filling machine packs the various liquids for pharmaceutical industry. It can handle the various packaging from vial filling, syrup filling, spray filling, eye drop filling and so on. The high request on the medical products like iodine, alcohol and so on can be satisfied by it. The great sanitary, large bulk and efficiency makes it outstanding in liquid filling of pharmaceutical industry.
Powder filling machine
ALLPAK Powder Filling Machine
Powder is also the frequently used medicine form in pharmaceutical industry. The powder filling machine packs various medical products like glucose, medical solids and so on. It can perform a series of work like separating, filling, locking and so on. For powder of various type and size, it can all handle.
Granule packaging machine
ALLPAK Granule Packaging Machine
Granule packaging machine can pack the various granule. You may see its frequent use in medicine-dissolved medicine and Chinese herb medicine. Granule is the important medical form in pharmaceutical industry. The granule packaging machine can assure the sanitary and efficiency of the whole packaging process.
Tablet packaging machine
ALLPAK Tablet Packaging Machine
Tablet packaging machine can count and pack various medicine form. And you may find the packaging of the various specification of tablets, capsules, soft gelatin capsules and other small medical devices. It can also make the mix packaging for nutrition pack. The great protection and showing of medical products make this packaging form so popular.
Capsule filling machine
AIPAK Capsule Filling Machine
Capsules are one of the most common medical dosage in pharmaceutical industry. Capsule filling machine should take the strict work to deal with different kinds and fine powder and granule. Capsule filling machine is a practical machine which can make the fluent and nice packaging work for various form and particles.
Tube filling machine
ALLPAK Tube Filling Machine
For pharmaceutical industry, there is the requirement for tube filling as the topical medicine like ointment and cream need the tube for accurate transferring. Tube filling machine makes the excellent work in packing ointment, pasta and various medical products into tube for pharmaceutical use.
Suppository filling machine
ALLPAK Suppository Filling Machine
Suppository can make the effective and useful delivering of medical effect for human. It makes great help for the patients who may be hard to take drug and medicines which are unsuitable for the stomach’s environment. Suppository filling machine makes the series of reliable work to make suppository with various shapes.
5.Importance Of Packaging Machine In The Pharmaceutical Industry
Packaging machine is irreplaceable in pharmaceutical industry and its importance cannot be underestimated. Here is the importance of packaging machine in pharmaceutical industry.
Accuracy and consistency
Consistency-Sourced:brainzmagazine
Packaging machines make the consistent and precise packaging. The right and precise packaging is rather important for various medical products. The wavering of the dosage in some kind may lead the change of effect. Packaging machines make the great job in making the accurate packaging which is needed for pharmaceutical industry.
Efficiency
Efficiency-Sourced:revechat
Packaging machines reduce the work of labor and make the packaging work with high efficiency. For the large production, it needs the high efficient packaging machine to make the timely production and make the organized managing. This can save their time and save more for business.
Automation
Automation-Sourced:mida
The high automation of pharmaceutical packaging machine helps reduce the human mistake and prove the sanitary on packaging process. The high automation pharmaceutical packaging machine take can also prove the high speed processing of the whole packaging process.
Quality prove
Quality Prove-Sourced:idexxcurrents
Packaging machine used in pharmaceutical industry can prove the products’ quality. The packaging process with little joining of human has smaller chance to be contaminated. And you may control the quality of the packaging products better from packaging machine rather than the labor work.
Regulatory compliance
Regulatory Compliance-Sourced:finreg-e
The pharmaceutical products are tested and inspected carefully as it makes so much important work for people. And the changing and advancing regulatory compliance needs the flexible packaging machine for pharmaceutical use. The adoption of packaging machine can improve traceability and offer reporting for the prove of products and business quality.
Better protection
Better Protection-Sourced:maidsbytrade
The packaging machines makes better packaging work. The packaging are made with series of strict packaging process and passes the test and check of packaging machine. The little adding of human and great packaging work can prove the better protection of machine in transporting and storing.
Anti counterfeiting
Anti Counterfeiting-Sourced:temera
The labeling and packaging work of packaging machines for pharmaceutical industry can make the great anti counterfeiting job. The tracing and reporting packaging machine offered is the great method for anti counterfeiting. For the labor work with low automation, the anti counterfeiting is impossible.
Cost saving
Cost Saving-Sourced:optimizemro
Packaging machines in the pharmaceutical industry is a big cost for pharmaceutical business. But in a long ran, it improves the packaging efficiency and improves the profitability. For the better qualified products made from pharmaceutical packaging machine, your business can make the better marketing work.
6.What Other Industry Can The Packaging Machine In The Pharmaceutical Industry Be Applied?
Packaging machines can be used in more than one places. What other industry can packaging machine be applied? Here are some representative industries which adopt the packaging machine frequently.
Food industry
Food Industry-Sourced:entrepreneurialchef
For food industry, there are also the high standard on food safety and packaging efficiency. The application of packaging machine in food industry can prove the quality of food. It can also make the various and attractive packaging which may promote sale in market.
Cosmetic industry
Cosmetic Industry-Sourced:somewang
Cosmetic products have also the the high sanitary request on cosmetic products packaging. The efficient packaging work can maintaining the efficiency of essence, lotion and other skin relative products. The reliable packaging can also prove the material which contact people’s skin.
Chemical industry
Chemical Industry-Sourced:gamerpackaging
The chemical products also have a wide application in our life. The chemical products have various form in our life and you may see iron, gas, plaints and so on. The packaging of chemical products should be clean and air tight for the pollution and deterioration for the further use.
Supplementary industry
Supplementary Industry-Sourced:trackmind
Supplementary industry is a blooming industry in current world. Vitamin, mineral, herb and so much supplementary products are waiting for the the clean and reliable packaging. You may see its various forms like tablet, capsule, pill, gummy and so on which all need the various packaging form for its nice transporting to market.
Agricultural industry
Agricultural Industry-Sourced:Packagingoftheworld
There are fertilizers, nutrition and so many other chemical products serving for agricultural industry. The packaging for agricultural industry should be stable and strong. The packaging form for agricultural industry should also be various for the various type. And you may see the packaging for tablet, powder and so on.
7.What You Should Consider For The Suitable Packaging Machine In The Pharmaceutical Industry Purchase?
Packaging machines for pharmaceutical use take the important role in pharmaceutical industry. But for so many different pharmaceutical packaging machine, what you should consider for the suitable machine purchase.
Cost
Cost-Sourced:hypergene
Cost is your first consideration for the suitable pharmaceutical packaging machine purchase. You should make clear your business’s condition and your business’s future developing road. The too much cost on pharmaceutical packaging machine purchase is a burden for your business rather than the help.
Product type
Product Type-Sourced:sgs
Tablet, capsule, powder or gummy, what is your product type? For different product type, you should get the relative machine. The powder filling machine should used for powder filling and eye drop filling machine for eye drop. The misuse of capsule filling machine for gummy packing may make big mistake.
Product capacity
Product Capacity-Sourced:mrpeasy
Product capacity is also the important factor for you to consider. The machine type can show machine’s capacity and performance. How much do your business need? Your machine should make the products which suit the need of your productivity. The type which is short in capacity can benefit your business no more.
Machine quality
Machine Quality-Sourced:highqualitymachine
Machine quality is the important factor for you to consider. The unqualified machine may make a series of mistakes in the manufacturing process and hinder your process. To choose the packaging machine with good quality, you should get the big brand and have a deep knowing about the machine judges, machine processing and machine quality.
Compatibility
Compatibility-Sourced:nsrd
You should investigate the features of your medical products with the different pharmaceutical machine. Will your packaging machine and packaging material affect each other? The compromising of the both can make the suitable and qualified products for your business.
Durability and sustainability
Durability And Sustainability-Sourced:amtonline
The products made from your pharmaceutical packaging machine should be durable and qualified for the long and complicated transporting and storing road. And your pharmaceutical packaging machines should also be able to make the sustainable products for the benefit of flexible packaging solution.
Automation line
Automation Line-Sourced:quickpakinc
Automation which improves the efficiency greatly is now the inescapable topic in our pharmaceutical industry. For the automated packaging line, you should make clear the automation level of your packaging machine for later automation packaging line forming.
Regulatory standard
Regulatory Standard-Sourced:fairinstitute
The medical products should get through series of inspecting for the suitable use. And there are various regulatory standard for different country and different medicines. To make your medicine circulate wide and smooth, you should make clear the local regulatory standard for the pharmaceutical packaging machine choose.
8.What Other MachineCan Support Packaging In The Pharmaceutical Industry?
After you have packaged your medicine products well, what else you should do? Your machine in this state cannot go on market yet. Here are the machines which can support your later pharmaceutical packaging.
Labeling machine
ALLPAK Labeling Machine
For better anti counterfeiting and propaganda, there should be label on medical products packaging. There are various labeling machine for you to choose and you may find the wrap-around labeling machine, automatic ampoule labeling machine, bottle labeling machine, double-sided labeling machine, top sticker labeling machine.
Cartoning machine
ALLPAK Cartoning Machine
The well packaged medical products need the tertiary packaging for the safer transporting and protecting in conveying and storing. The cartoning machine is thus essential equipment for the further support of your pharmaceutical packaging.
Flow wrapping machine
AIPAK Flow Wrapping Machine
You may find the plastic filming seal for your well packaged products while you shopping in market. The flow wrapping machine give your packaged medical products the direct protecting from dust or light. It can also support your pharmaceutical packaging work in many way.
9.What Is The Future Trend Of Packaging Machine In The Pharmaceutical Industry?
The packaging machines in the pharmaceutical industry are made more and more advance in current time. And there are the clear future trend for the packaging machine in pharmaceutical industry.
Customization
Customization-Sourced:istockphoto
The future packaging machine in pharmaceutical industry should be customized for the various packaging products and packaging performance need. The customized packaging machine should be highly efficient and automated for the dealing with various kinds of products.
Intelligence
Intelligence-Sourced:nubrickpartners
The intelligence and automation of packaging machine can be further updated. The joining of people in packaging process will be less. The high automation with great intelligence can make the great work which improves efficiency and saves labor working.
Sustainability
Sustainability-Sourced:businessreporter
In future pharmaceutical packaging, the concerning on sustainable and eco-friendly will be greater. And your packaging solution should also be sustainable and eco-friendly for the environment and safer use.
Conclusion
The importance of packaging machine in the pharmaceutical industry is obvious. The selection of the packaging machine which suits your pharmaceutical business can benefit you in an all around way. If you have any problem or questions, do not hesitate to contact ALLPAK now.
Don't forget to share this post!
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 7101 8586
The Buyer's Guide

Importance of Packaging Machine in the Pharmaceutical Industry Read More »







































































 Tell us your material or budget, we'll reply you ASAP within 24 hours
Tell us your material or budget, we'll reply you ASAP within 24 hours















































 Tell us your material or budget, we'll reply you ASAP within 24 hours
Tell us your material or budget, we'll reply you ASAP within 24 hours