Installation Qualification Example: The Complete FAQ Guide In 2025
Are you trying to install equipment? Are you trying to minimize hazardous risks during installation? Installation is a must before successfully operating the equipment. Effective installation guarantees the smooth performance of any device. One must follow manufacturer specifications and recommendations to ensure error-free installation. How to verify that the installation conforms with standards?
Installation qualification is an indispensable procedure that ensures the instrument and systems’ safety, quality, functioning, and reliability. It is not only a good practice but also a legal requirement. People are always confused about the definition of installation qualification and what it entails. So, here is a FAQ guide “Installation Qualification Example: The Complete FAQ Guide In 2024” to satisfy ever-curious minds. Let’s look at the list of questions.
1.What is installation qualification?

Installation Qualification- Picture Courtesy: Zamann Pharma
Installation qualification is a module of the process validation protocol that comprises a documented series of steps to assure manufacturers and regulatory bodies of effective delivery, installation, mounting, calibration, and arrangement of devices and systems.
It is the first step in the equipment qualification protocol and records every vital facet of equipment installation and verifies that these installation steps are carried out by following guidelines defined in the design specification, equipment manual, and in-facility user requirements.
Installation qualification provides documented evidence for the correct installation of the instrument and ensures that its installation will not severely impact its functioning. It verifies the installation and configuration of the machine, its components, piping, or service against a manufacturer requirement checklist. This step is performed before operational and performance qualification.
2.What is an installation qualification example?

Installation Qualification Example- Picture Courtesy: GF Machining Solutions
Whether your instrument is physical or digital, once you have configured it, here are some installation qualification examples for you to inspect your installation protocol:
| Location Plan | Find a suitable location and ensure its suitability regarding floor space, ventilation, and outlets or drainage system. |
| Utility Inspection | Verify that various utilities, for example, water, electricity, compressed air, or other facilities are mounted as per design drawings. |
| Documentation | Collect and review every manual, certification, instruction guide, checklist, and other to verify the validity of every installation step. |
| Unpacking | Unwrap every piece of instrument and inspect them for wear and tear. Cross-check every step with the manufacturer's guide to ensure you are proceeding in the right way. |
| Component Verification | Install ancillary parts and other components, for instance, sensors, seals, screws, valves, etc. Ensure that they are fitted and aligned properly. |
| Recording | Note details like firmware models, serial numbers, and other instrument-related information. |
| Inspection of Environmental Setting | Examine and ensure that environmental and functioning conditions, for instance, temperature, humidity, and ventilation are according to manufacturer recommendations. |
| Software Installation | Verify that the updated version of the software is installed in the desired location. Check that the software is set up with correct settings and network accessibility and performs its core functions as anticipated. |
| Calibration | Carry out every component calibration or adjustment. Record these calibration procedures with their execution dates and tools. |
| Connectivity Validation | Check every connection and communication is established with secondary or supplementary support components in line with the user guide. |
3.How does the installation qualification example serve industries?
Installation qualification example has central importance in ensuring performance and conformity of equipment and systems. It is a critical testing phase that has immense value in verifying the quality and efficiency of procedures in regulated manufacturing units. Let’s detail its important uses:
Compliance with Regulations
Compliance with Regulations
This process is a pivotal requirement for industries overseen by regulatory authorities, such as the FDA, EMA, or, WHO. It confirms that instruments are installed according to configuration parameters set by the manufacturers and governing bodies. Its records are typically inspected during regulatory audits. Therefore, fulfilling the installation qualification example is a failsafe way to meet GMP and ISO quality standards.
Risk Alleviation
Risk Alleviation- Picture Courtesy: PharmOut
This testing validation minimizes risks associated with incorrect installation of the instruments. Improper system installation and configuration could lead to erroneous measurements and faulty treatment procedures. Thus, the installation qualification example mitigates problems related to system malfunctioning, failure, and safety incidents.
Instrument Longevity
Instrument Longevity
The installation qualification example is a reliable approach to monitoring minor errors in the installation process that contribute to problems down the line and affect the working of the system. The rightly configured instrument generally has a lower component breakdown and lasts for many decades.
Records for Auditing
Records for Auditing
The documentation comprising installation qualification examples is a historical record of installation procedures and inspection checklists. It also documents details of operators checking installation parameters and errors that are rectified during the installation step. This report is integral for auditing productions and helps in troubleshooting technical issues that may arise in the future.
Decreasing Downtime
Decreasing Downtime
The installation qualification example serves as a guide for technicians to properly configure the machine first time. So, operational delays and expensive downtime are prevented by following correct installation standards. This also cut down the need for recurrent machine alteration and repairs, saving maintenance costs and operational time.
4.What type of industries need the installation qualification example?
Installation qualification is integral in laying the groundwork for process validation. Since it ensures the stable and effective working of the instruments; therefore, it is needed in various industries, including:
Pharmaceutical Industry
Pharmaceutical Industry- Picture Courtesy: Verywell Health
A wide range of equipment is utilized in the pharmaceutical industry to manufacture top-quality, safe, and potent dosage forms. Pharmaceutical machines, for instance, mixers, blenders, tablet presses, capsule fillers, granulators, etc., must be validated using an installation qualification example checklist to ensure they are fit for working and operate according to expected criteria.
Biotechnology Industry
Biotechnology Industry- Picture Courtesy: BioSpectrum India
Installation qualification example is a proactive step to reduce the adverse risks linked with the installation of biotech equipment, for example, incubators, centrifuges, chromatography devices, spectrometers, and bioreactors. This measure ensures that biotech systems are qualified for their proposed purpose. Therefore, it is indispensable in biotechnology industries for the efficient validation of research protocols and bioengineered products.
Medical Industry
Medical Industry- Picture Courtesy: Asimily
In the medical industry, the installation qualification example serves as an indicator of the safety and quality of medical devices and products. Proper installation of medical instruments- for example, sterilization units, diagnostic tools, surgical devices, etc.- is essential in patients’ treatment and safety. Any discrepancy in the installation of medical devices can jeopardize the life of the patient.
Food and Beverage Industry
Food and Beverage Industry- Picture Courtesy: Kompass Solutions
The installation qualification example is not just a regulatory requirement in the food and beverage industry; it is a significant measure to guarantee consistency in the food and beverage sector. It satisfies the safety and regulatory standards set by HACCP and ISO 22000 for the food industry. By following correct installation qualification examples, brands decrease contamination during manufacturing and build customer trust in the quality of their produced goods.
Chemical Industry
Chemical Industry- Picture Courtesy: Sigma-Aldrich
Different types of instruments- such as chemical reactors, distillation units, pumps, and heat exchange systems- are routinely used in the chemical industries. So, to comply with OSHA, EPA, RCRA, and, NPDES, the chemical manufacturers must perform installation qualification protocol. This validation protocol verifies that chemical equipment is safely installed and does not pose any risk to the operator and environment.
Cosmetics Industry
Cosmetics Industry- Picture Courtesy: More Natural
Compliance with GMP protocols is the first step to successfully marketing and distributing cosmetic products. Deviations in quality assurance steps directly affect the user's health. So, cosmetic brands follow recommended installation qualification examples to verify that their cosmetic products are designed by the highest quality standards.
Electronic Industry
Electronic Industry- Picture Courtesy: Integra Sources
Proper installation of manufacturing units- such as cleanrooms, water assembly systems, and testing tools- is pivotal in electronic and semiconductor fabrication. Thus, an installation qualification example is needed in this industry to verify manufacturing precision and prevent risks like electronic failure and fire incidents.
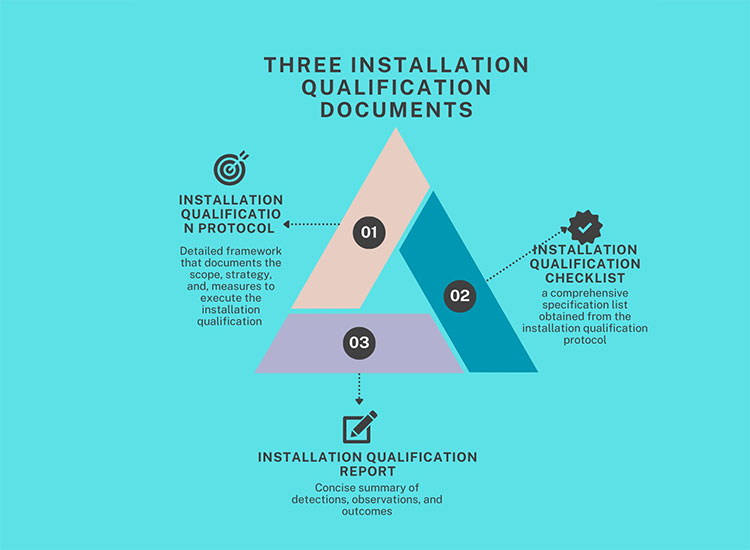
5.What are fundamental installation qualification example documents?
Installation qualification example is a prerequisite of any validation process and to confirm that it proceeds as required, one must have basic installation qualification example documents, including:
Fundamental Installation Qualification Example Documents
Installation Qualification Protocol
Installation Qualification Protocol
It is a detailed framework that documents the scope, strategy, and, measures to execute the installation qualification example. It further details:
- Instrument identification essentials (version, product code, tag, manufacturer, vendor).
- Name of devices and systems to be qualified.
- Installation needs specified according to the manufacturer's guide.
- Environmental conditions for installation.
- A worksheet of approval criteria
Installation Qualification Checklist
Installation Qualification Checklist
It is a comprehensive specification list obtained from the installation qualification protocol, encompassing every facet of installation validation. It covers manual component inspection, utility installation, electric connections, calibration, hardware and software installation checkpoints, alignment, screw fastening, installation of axillary parts, and control schematics.
Installation Qualification Report
Installation Qualification Report
In this report, details of steps performed during the execution of the installation qualification protocol are mentioned. It offers a concise summary of detections, observations, and outcomes. Moreover, it explicitly expresses the acceptance or rejection of instrument installation criteria.
6.How do you fulfill the installation qualification example, step by step?
Installation qualification example is a structured procedure, requiring careful planning and a proper mindset. It can be subdivided into five major steps to achieve precise system validation. Let’s discuss step by step completion of installation qualification example:
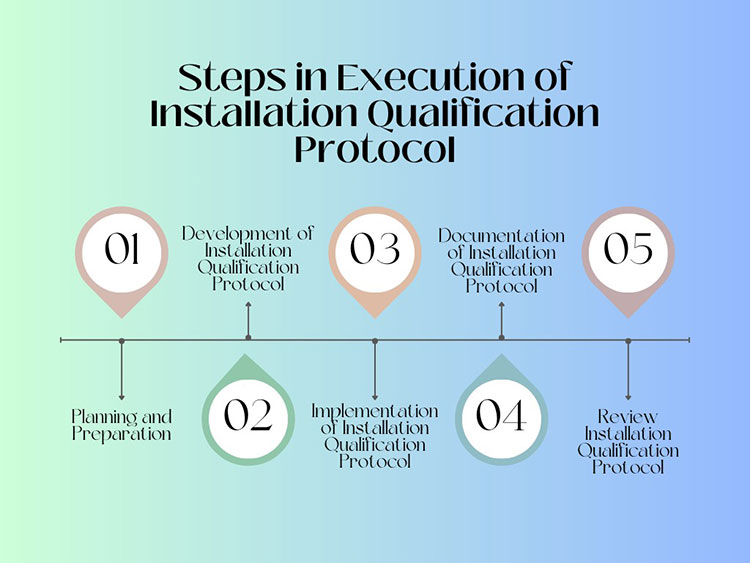
Steps in Execution of Installation Qualification Protocol
Step 1: Preparation and Planning
Planning of Installation Qualification Protocol- Picture Courtesy: Dickson Data
It is crucial to prepare and plan by reviewing objectives and establishing the scope of installation qualification before starting installation tests. You should define what instruments and parts will be tested and make sure all the applicable facilities are available for installation qualification.
You should also specify how to handle any issue encountered during the qualifying procedure. Similarly, identify and assess perils associated with installation. This is critical to measure the severity and probability of risk and develop suitable risk alleviation approaches.
Step 2: Development of Installation Qualification Protocol
Development of Installation Qualification Protocol
After executing the preparation step, you should develop the installation qualification protocol. This code of conduct should mention the instrument or system, the name of the qualifying test, its methodologies, approval standard, rejection criteria, test outcomes, testing personnel, and date of testing.
It should enlist details of qualification testing to be performed, including assessment. It should specify the jobs, tasks, and responsibilities of personnel who will conduct the qualification process and who will evaluate and accept results. Moreover, references to documents used using qualifying procedures, variances handling, and modification adjustment procedures should be mentioned in the protocol.
Step 3: Implementation of Installation Qualification Protocol
Implementation of Installation Qualification Protocol- Picture Courtesy: Overbook Scientific
The skilled personnel carry out stated tests and examinations during this step to verify that the installation satisfies the preset approval criteria. In the execution phase, the proper installation of the instrument is verified, the calibration of devices is inspected, and the working of control panels is evaluated.
It is pivotal to adhere to the qualification protocol diligently and record the outcomes precisely. Also, any variances and abnormalities during testing should be documented and addressed accordingly. In this way, you can ascertain the reliability and traceability of qualification validation testing.
Step 4: Maintain Records of Installation Qualification Protocol
Maintain Records- Picture Courtesy: Freezerworks
Documentation is a key aspect of the installation qualification example because it offers records of testing tasks and verification of compliance, thus leading to simpler future inspections. It is recommended to document every piece of information like test figures, annotations, conclusion, installation details, qualification methodology, etc. in an explicit and systematized manner during the complete duration of the documentation phase.
This documentation must be detailed, precise, and readily available. This step also includes keeping version changes, utilizing regulated templates, and housing documents in a safe and centralized area in the facility.
Step 5: Review Installation Qualification Protocol
Review Installation Qualification Protocol- Picture Courtesy: Dickson Data
The last step in the installation qualification example is to assess and accept test outcomes and ancillary documentation produced during the qualification procedure.
The authorized personnel should inspect the qualification outcomes thoroughly to attest every prerequisite has been satisfied and if the installation is appropriate for its expected task.
In addition, they should meticulously read the documentation. They should attest that every investigation and examination is performed accurately, that approval criteria were satisfied, and any installation errors are rectified successfully.
7.What are ideal practices for the effective installation qualification example?
The installation qualification example should be executed diligently to avoid any inconsistencies during qualifying tests. So, here are some ideal practices that should be kept in mind to successfully carry out the installation qualification example:
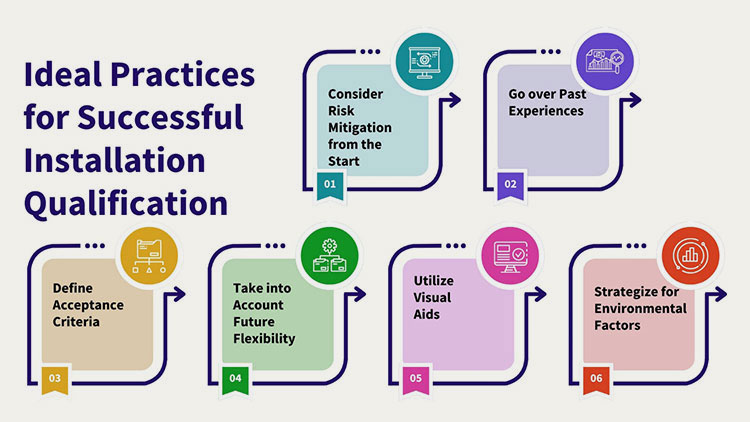
Ideal Practices For The Effective Installation Qualification
Consider Risk Mitigation from the Start
Consider Risk Mitigation from the Start- Picture Courtesy: Soluen Engineering Solutions LTD
It is important to integrate a risk-grounded strategy early on into the installation qualification protocol for recognizing probable risks related to instrument installation and prioritize qualification strategies based on these risks. By this personnel can ensure that serious problems impacting product quality and safety are resolved first.
Go over Past Experiences
Go over Past Experiences- Picture Courtesy: ZipRecruiter
Review old installation protocols and documentation for the same device within the facility. This review can uncover frequent installation problems, which leads to proactive modification within the protocol.
Define Acceptance Criteria
Define Acceptance Criteria
Instead of specifying that the instrument must be configured according to manufacturer guidelines, you should precisely describe your quantifiable and specific approval criteria for every roadblock in installation. It clears any vagueness and ascertains that validation auditors empirically evaluate compliance.
Take into Account Future Flexibility
Take into Account Future Flexibility- Picture Courtesy: 35 North
It is best to plan the installation qualification protocol by considering future instrument upgrades or adjustments. A modular section in documentation helps in quick and effortless updates, which decreases the necessity of rewriting the whole document later on.
Utilize Visual Aids
Utilize Visual Aids
Ideally, illustrations, flowcharts, and, pictures should be included in the installation qualification example protocol to improve the clarity of installation guidelines and expectancies. This aids in easier understanding and implementation from technicians and validation teams. Visual aids- for example, comprehensive wiring figures- guide technicians and decrease installation errors.
Strategize for Environmental Factors
Strategize for Environmental Factors- Picture Courtesy: Renejix Pharma Solutions
Consider and plan for the effect of environmental settings (temperature, humidity, air) on installation steps. Incorporate any expected environmental controls- like keeping the temperature under the required limit – and inspections to guarantee that these conditions do not severely impact the equipment installation and operation.
8.How to troubleshoot frequent errors during installation qualification example?
Experienced people admit that installation qualification is a challenging job. Resolving these challenges is a crucial job in the validation process to ensure that the instrument is correctly installed, otherwise next stage of validation does not occur.
So, let’s discuss these frequent errors and their solutions during the installation qualification example:
Improper Utility Connections
Improper Utility Connections
The instrument does not start because of an incorrect or insufficient connection with electricity, water, gas, etc.
Troubleshooting
Look through the manufacturer’s guide for electric current, pressure, and, water flow to ascertain these utilities are properly connected. Manually examine connection points to verify they are connected with matching inlets or outlets. To test gas lines or compressed air connections, it is advised to use a pressure gauge. Moreover, a multimeter should be utilized to assess electric assembly. The utility diagram of the factory or laboratory should match with equipment requirements, if not then update it.
Component Misalignment
Component Misalignment
Sometimes, during the installation, mechanical parts, for instance, drives, sensors, conveyor belts, pressure valves, and more are incorrectly aligned or assembled.
Troubleshooting
First, physically locate any misaligned or loose components, for instance, belts, and gears, then dismantle those parts, and reinstall them correctly and tightly as per manufacturer specifications. It is advised to use precision orientation devices like a ruler, dial indicator, and laser leveling tool to correctly position and level components. Inspect different bolts, screws, nuts, and other rivets, to properly secure them.
Inaccurate Calibration
Calibration in Installation
Different accessory devices like temperature sensors, pressure gauges, piston gauges, and gas piston meters have inadequate, or unverified calibration ranges. Sometimes, these instruments are found out of calibration during installation qualification.
Troubleshooting
To address this challenge, you should first look for valid calibration certifications for every vital piece of equipment and if they are outdated then renew their calibration. If required, you must carry out in-house calibration with standard calibration reference. Also, document every calibration outcome in the installation qualification protocol and note any anomaly and actions performed to encounter during this process.
Unsuccessful Safety Assessment
Unsuccessful Safety Assessment- Picture Courtesy: Fluke Corporation
During installation qualification, there comes a time when safety components, such as emergency stop buttons, alarm lights, interlocks, gaskets, and valves fail to meet safety criteria because they are non-functional or incorrectly fitted.
Troubleshooting
To rectify this installation error, inspect wiring and other connections of different safety features, and verify that they are wired and assembled properly according to the manufacturer's standards. Activation of safety instruments should be checked by inducing unsafe settings in the facilities. Replacement of faulty instruments must be carried out with certified components. Last but not least, it is suggested to contact the manufacturer for troubleshooting support if the device still fails to work after following the above instructions.
Labeling Slipups
Labeling Slipups- Picture Courtesy: Marcajes Telleria
Systems or other crucial devices are not correctly labeled, or have missing identification tags, causing mix-up during processing or maintenance.
Troubleshooting
It is recommended to solve this issue by going along with these suggestions like cross-checking the labeling stipulations from the installation qualification with manufacturing standards. Place correct, long-lasting, and, clear labels or tags on every part, utility, wiring, or piping. Installation qualification documentation should be updated to indicate precise labeling items for the upcoming review.
Conclusion
Installation qualification example in an integral procedure in the validation process to confirm proper installation, configuration, and functioning of any system, equipment, component, and, software. By following the step-by-step instructions and complying with the best practices mentioned in this blog post, you can efficaciously and lucratively conduct installation qualification examples, mitigate installation and operational mistakes, and satisfy stringent regulatory standards for your business and facility. Now, if you are keener on learning about installation qualification protocol for validation of routine industrial devices, you are highly encouraged to contact Allpack through our messaging or email service.
Don't forget to share this post!
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 7101 8586
The Buyer's Guide
 Tell us your material or budget, we'll reply you ASAP within 24 hours
Tell us your material or budget, we'll reply you ASAP within 24 hours