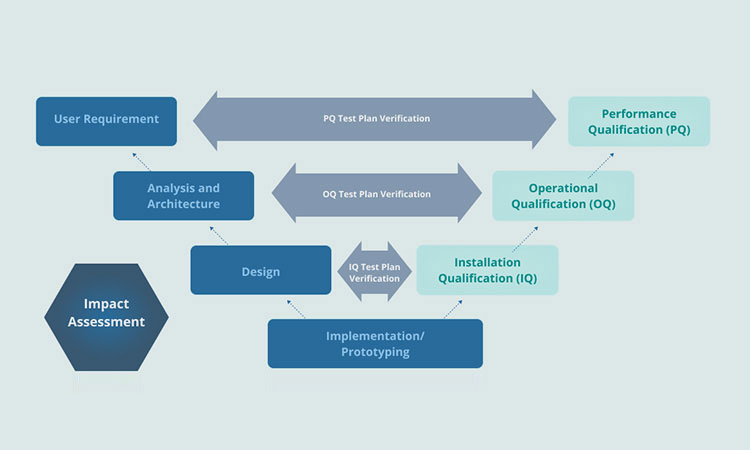
Performance Qualification VS Operational Qualification
In the pharmaceutical and clinical industries, even a slight mistake can lead to serious problems. Therefore, in the pharmaceutical, medical device and clinical industries, they should be strictly regulated. In order to ensure the safety and quality of equipment in production and operation, operational qualification and performance qualification are proposed and strictly stipulated.

Performance Qualification VS Operational Qualification-sourced: csolsinc
What is operational qualification and performance qualification? Why they are important? How to perform and what you need to pat attention? Following this performance qualification vs operational qualification post, you may grab the free guide. Let's go!
1.What Is Operational Qualification?
What Is Operational Qualification-sourced: tgm
Operational qualification refers to a series of tests on equipment. Its purpose is to determine whether the equipment performance meets the user's requirements and specifications within the operating range specified by the manufacturer. This type of testing has specific requirements and specifications.
After testing, the performance of the equipment will be recorded. It includes a detailed review of the equipment startup, operation, maintenance, cleaning and safety procedures of the equipment hardware and software.
2.What Is Performance Qualification?
What Is Performance Qualification-sourced: Pharmaguideline
Performance qualification is the next step after operational qualification. It is the final stage of equipment certification. Performance qualification does not test the components and instruments of the equipment separately.
It generally introduces a third-party expert to ensure the thoroughness and accuracy of the equipment. The purpose of this process is to ensure that the product quality of the equipment is continuously guaranteed during its operating time.
3.Performance Qualification VS Operational Qualification: How To Perform?
Unlike operational qualification, performance qualification does not test individual parts of the equipment.
How To Perform Operational Qualification-sourced: Pharmaceutical Specifications
| Operational Qualification: How To Perform | |
| Perform functional tests | l Perform tests to verify that the equipment's operating functions are within the specified range. |
| Safety measures check | l Test all safety functions of the equipment, including alarms and fault protection, to ensure that the equipment is in normal working condition. |
| Validation plan development | l Record the complete equipment test performance and data, and analyze the deviation from the expected parameters. |
| Report generation | l Compile all data reports into a comprehensive operational qualification report in accordance with specifications and requirements for subsequent retrieval and review. |
How To Perform Performance Qualification-sourced: gmpinsiders
| Performance Qualification: How To Perform | |
| Unify performance standards | l Clear and unify the performance standards of the equipment under operating conditions. |
| Long-term equipment testing | l Run the equipment for a long time to record and evaluate its long-term performance. |
| Environmental condition testing | l Run the equipment in different environments and conditions to record and evaluate its performance. |
| Data analysis | l Verify, correct and re-test the different differences shown by the equipment under different environments and conditions. |
| Report generation | l Compile all data into a detailed performance qualification report for subsequent retrieval and review. |
4.Performance Qualification VS Operational Qualification: What Makes Them Successful?
After the operational qualification and performance qualification are performed, you may know whether they are successful through the following standards and indicators. They are:
OQ
OQ-sourced: nodeviation
The equipment can operate normally within the operational range;
- All operational tests meet the acceptance criteria of the operational qualification protocol;
- During the test, the equipment operation has no deviations or unqualified conditions;
- During the test, the deviation of the equipment operation will not affect the overall capability or other performance of the equipment;
- The equipment can correctly identify and handle faults and errors;
- It can connect other integrated equipment without any compatibility issues or interruptions;
- Positive operator and user feedback;
- The operational qualification test is successfully completed and all standards are met.
PQ
PQ-sourced: media
- The equipment's products and product processes meet the standards;
- The equipment's process capabilities are stable and meet the standards for a long time;
- The equipment's system can handle multiple products without lag problems;
- The work processes will not affect each other;
- The equipment can correctly identify and handle different known materials;
- The equipment can operate normally and meet the relevant standards in actual work;
- The equipment can automatically identify some potential unqualified conditions during operation, including: overheating, excessive vibration, noise, pressure difference, process medium back-flow, etc.
5.Performance Qualification VS Operational Qualification: Why You Should Perform PQ And OQ?
Why should you obey and perform the operational qualification and performance qualification? In short, this is the FDA's requirement. To expand, its benefits are:
OQ
Reasons to Perform Operational Qualification-sourced: polarseal
Reduce risks
Obtaining operational qualification in advance can reduce the risk that the equipment cannot operate normally or as expected, thereby it can reduce the loss of users.
Protect equipment parts
If the equipment does not pass operational qualification, it may face risks such as component damage, software problems, and workflow problems. After operational qualification, these problems can be well eliminated and parts can be protected from damage.
Help manufacturers improve
Only through a series of tests in operational qualification can equipment manufacturers find problems and make corresponding improvements. Therefore, the upper and lower limits of the equipment can be determined and the corresponding operating standards can be made.
PQ
Reasons to Perform Performance Qualification-sourced: polarseal
Eliminate potential equipment problems
The hardware and software problems of the equipment may meet the standards, but it is difficult to find other potential problems without operating tests. For example, overheating, excessive vibration, noise, pressure difference, etc.
Ensure equipment performance
Through performance qualification testing, the normal and stable operation of the equipment can be guaranteed, thereby ensuring the performance of the equipment for a long time.
Forming validation documents
If any problems occur during the use of the equipment, the verification documents of performance qualification can be used to eliminate the problems in the operation of the equipment and help users, manufacturers or regulatory agencies to find the cause.
6.Performance Qualification VS Operational Qualification: What Are Their Essential Documentation?
When the equipment has passed the operational qualification and performance qualification, a comprehensive document will be formed. It includes:
OQ Documentation
OQ Documentation-sourced: golighthouse
Operational qualification documentation mainly includes the equipment's objectives, scope, methods, acceptance criteria, requirements, and reports.
Objective: clarify the equipment's objectives, specific operating parameters and functions that need to be tested.
Scope: equipment-related components, systems, hardware, software, and operating procedures;
Methods: equipment operating steps and procedures, related conditions and settings, etc.;
Acceptance criteria: what conditions the equipment needs to meet to pass each test;
Requirements: manufacturer and user specifications, supervision of equipment, etc.;
Report: OQ report mainly records the execution and test results of the equipment, including whether it is faulty, whether there is deviation, etc.
SOP: mainly includes equipment operation, maintenance, calibration, processing, recording deviation and other procedures.
PQ Documentation
PQ Documentation-sourced: golighthouse
Performance qualification documentation mainly includes equipment pre-qualification inspection, program testing, data collection, acceptance criteria, deviation management, document formation, document approval and review.
Pre-qualification inspection: ensure that the equipment has been properly installed and calibrated, and properly maintained;
Procedure testing: ensure that the content and process of the equipment's system procedure testing are fully executed;
Data collection: determine the data collection method and ensure that the data collection tools and systems are operating normally;
Acceptance criteria: clarify that the equipment's acceptance criteria meet industry standards and regulatory requirements;
Deviation management: debug, correct, record and retest equipment with data deviations;
Documentation: ensure that all required documents are complete and correct;
Approval and review: requires review and approval by relevant personnel.
7.Performance Qualification VS Operational Qualification: What Are Their Reports?
Compared with operational qualification documentation, its reports are simpler. It is mainly used to verify whether the equipment system, software or hardware operates as expected.
OQ reports
OQ reports-sourced: biotechserv
- List of test items for equipment acceptance criteria;
- Documentation for executing equipment test runs;
- List of information about equipment test items and sequences;
- List of designated equipment test tools;
- List of equipment acceptance criteria;
Performance qualification reports are the final documents for the entire equipment. It indicates that the equipment has passed the test and meets all standards.
PQ reports
PQ reports-sourced: dicksondata
Test summary: including an introduction and objectives of the equipment test;
Summary of test content: detailed description of the equipment execution process, including test methods, environmental conditions and test results;
Data analysis: the analysis and improvement of data deviations and qualification process;
Test conclusion: summary of test results;
Test recommendations: recommendations for improvement and adjustment of subsequent equipment;
Approval: signature of test and responsible personnel;
8.Performance Qualification VS Operational Qualification: What Are The FDA Requirements?
FDA attaches great importance to the qualification process and it is part of its regulation. The purpose is to allow drugs and medicines to be manufactured under normal and controlled conditions. Their requirements are:
Comply with good manufacturing practices
Comply with good manufacturing practices-sourced: eriks
FDA requires that devices should follow good manufacturing practices. This includes accurate documentation, precise data collection, and performance testing that meets standards.
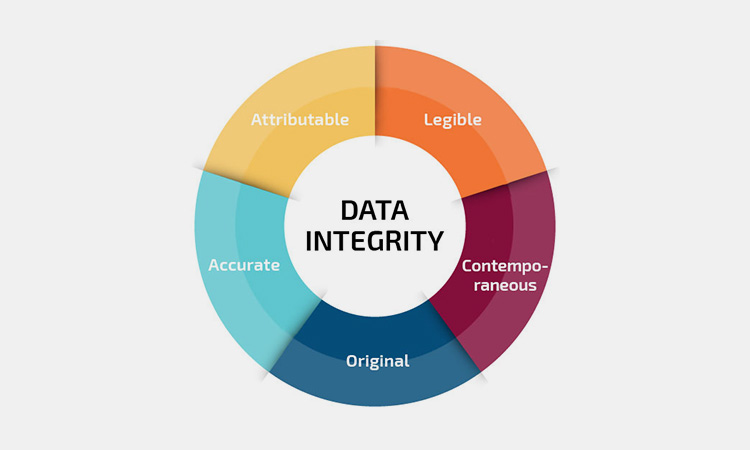
Data integrity
Data integrity-sourced: fivevalidation
Whether you perform operational qualification or performance qualification, you need to ensure the accuracy, consistency, authenticity, and reliability of the test data during the test process. If there are any deviations, they need to be marked and explained.
Document compliance
Document compliance-sourced: docsvault
You need to archive the qualification process and related documents to facilitate audits and supervision by subsequent staff.
9.Why Is Performance Qualification And Operational Qualification Critical In The Pharmaceutical Industry?
The operational qualification and performance qualification are critical in the pharmaceutical industry rather than other industries. Reasons are:
Regulations of the U.S. Food and Drug Administration (FDA)
Regulations of the U.S. Food and Drug Administration (FDA)-sourced: thefdagroup
This is a hard requirement of the FDA. The FDA believes that equipment certification is essential for the production and production of drugs. Any manufacturer needs to ensure that their equipment is well designed, easy to clean and maintain, and has the prescribed effectiveness and suitability.
Product quality assurance
Product quality assurance-sourced: sofeast
Only equipment that has passed operational qualification and performance qualification can its quality be guaranteed. And it can help drugs to be produced safely and accurately.
Patient safety assurance
Patient safety assurance-sourced: encrypted
Even the slightest equipment installation error may cause the produced drugs to have fatal consequences for patients. Only by ensuring the production and operation safety of the equipment can the safety of patients be guaranteed.
Help record and track
Help record and track-sourced: integrity-asia
Even if the equipment has passed operational qualification and performance qualification, there is a chance of problems in the later operation. Therefore, the relevant test records can well help users troubleshoot, record and track.
10.What Types Of Tests Are Conducted During Performance Qualification And Operational Qualification?
There are specific tests need to be conducted during the operational qualification and performance qualification.
The main OQ test types are:
The main OQ tests-sourced: media
Functional test: mainly checks whether the equipment operates as expected;
Stress test: evaluates the operating effect of the equipment under extreme conditions;
Calibration check: ensures the accuracy and stability of the equipment;
Repeatability test: takes the average of multiple running results to evaluate the stability of the equipment.
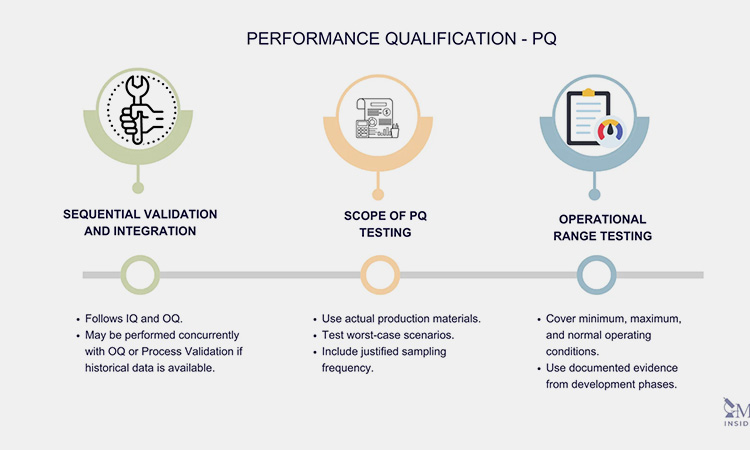
The main PQ test types are:
The main PQ tests-sourced: susupport
Operating range test: evaluates the upper and lower limits of the equipment;
Calibration and verification check: compares and verifies with known standards to ensure accuracy and consistency;
Sample analysis: tests samples produced during the operation of the equipment to check whether their quality meets the standards;
Integration test: connects with other whole-line equipment to ensure the smoothness and stability of the whole line.
11.What Happens If Performance Qualification And Operational Qualification Test Failed?
Operational Qualification and Performance Qualification Failed-sourced: chapter5academy
If the operational qualification and performance qualification tests fail, the reason may be that the test results did not achieve the expected effect, or the data produced a very large deviation. This equipment may need to be adjusted, repaired or re-qualified. The operational qualification and performance qualification can be re-qualified by re-adjusting the equipment.
12.What Are The Best Practices For Writing Performance Qualification And Operational Qualification Protocols?
After passing the operational qualification and performance qualification, you need to write the corresponding qualification protocols. You can follow the practices below to do this.
Practices For Writing Operational Qualification Protocols
Operational Qualification Protocols-sourced: madgetech
Operational Range
Normal environment testing, upper and lower limit environment testing. For example, you can test the device at the highest speed and the lowest speed range. This ensures that the device maintains stable performance during variable production requirements.
Failure Mode Testing
Test the device's ability to detect and handle fault conditions by simulating fault scenarios. This evaluates the reliability of the device's protection measures and management system.
Interoperability Testing
Connect the device to other devices or other complete lines to test the device's interoperability. To ensure seamless connection and online between systems.
Execution Sequence Testing
Have the device operate the test as required and record the execution sequence. This can reflect the use of the device during the production cycle, ensuring a smooth and efficient production workflow.
Load Testing
Load the device under different conditions, including maximum capacity and variable load. This ensures that the quality and reliability of the device can be maintained under load.
Practices For Writing Performance Qualification Protocols
Performance Qualification Protocols-sourced: madgetech
Real-time Monitoring
Use real-time monitoring tools to analyze and summarize the real-time data provided during the operation of the equipment for subsequent adjustments.
Dynamic Adjustment
Combined with the application of process analysis technology, dynamic adjustment of parameters is performed.
Scalable Protocols
Design scalable protocols. Make sure that the protocols you design are scalable. This will facilitate the subsequent test process to expand the production scale.
13.Performance Qualification VS Operational Qualification: What Is An Equipment Qualification Protocol?
Equipment Qualification Protocol-sourced: egnyte
The equipment qualification protocol is a written plan. It describes the qualification process of the equipment. This protocol mainly includes the component evaluation of the equipment, the steps of OQ and PQ qualification, parameter testing, product testing, and the production process and deviation handling.
Step 1
Write and formulate the protocol. A detailed document is prepared by relevant staff or experts. It mainly includes the scripts, methods and steps you need to test. Standardize acceptable test results. Specify relevant parameters and acceptance criteria.
Step 2
Prepare the pre-checklist. Evaluate the system for equipment inspection and testing to ensure product quality and user safety. Establish and divide the corresponding time and responsibilities.
Step 3
Thoroughly understand and inspect the equipment. Understand the intended use and effect of the equipment and recognize the functions of the equipment.
Step 3
Test the equipment according to the requirements and specifications. And collect the necessary documents and data. Handle it according to the design specifications and standard operating procedures.
Step 4
Understand the documents and processes required for certification OQ and PQ. Understand the overall process flow of the equipment.
Step 5
Review the FAT and SAT records that have been carried out. Conduct evaluation and analysis.
Step 6
Thoroughly review current regulatory guidance and warning letters. Develop clear and concise protocols.
Conclusion:
Operational qualification and performance qualification are key processes in the pharmaceutical industry. In addition to enabling equipment to operate continuously and effectively, they also ensure product compliance, high quality, and safety. Through this complete performance qualification vs operational qualification guide, you can also follow such a guide later. If you have more questions, you are welcome to consult ALLPACK now!
Don't forget to share this post!
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 7101 8586
The Buyer's Guide
 Tell us your material or budget, we'll reply you ASAP within 24 hours
Tell us your material or budget, we'll reply you ASAP within 24 hours